-
Aquestive Therapeutics Reports Positive Topline Data from Part 1 of EPIPHAST Trial Evaluating AQST-109 Epinephrine Oral Film
来源: Nasdaq GlobeNewswire / 25 2月 2022 06:00:00 America/Chicago
- First and only orally delivered epinephrine product candidate AQST-109 continues to show rapid absorption with a median time to peak concentration (Tmax) of 13.5 minutes
- AQST-109 was safe and well tolerated
- Part 2 of the EPIPHAST trial commenced
WARREN, N.J., Feb. 25, 2022 (GLOBE NEWSWIRE) -- Aquestive Therapeutics, Inc. (NASDAQ: AQST), a pharmaceutical company advancing medicines to solve patients’ problems with current standards of care and provide transformative products to improve their lives, today announced positive topline results from Part 1 of the EPIPHAST study for its AQST-109 epinephrine oral film. Part 1 showed that key pharmacokinetic measures were aligned with previous favorable results for AQST-109 and that the product was well tolerated with no serious adverse events. A lead candidate is currently being evaluated in Part 2 of the EPIPHAST study.
EPIPHAST is a randomized, open-label, three-part adaptive design, crossover study in healthy adult subjects comparing the pharmacokinetics and pharmacodynamics of epinephrine delivered via Aquestive’s AQST-109 oral film compared to intramuscular injection of epinephrine. The study is being conducted pursuant to clearance from Health Canada.
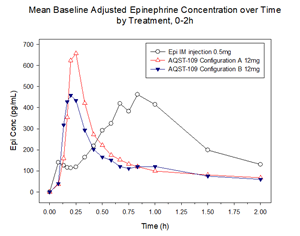
In Part 1 of the EPIPHAST study, multiple oral film formulations and dosage strengths of AQST-109 were evaluated. As shown in the figure below, the lead formulation of AQST-109 rapidly reached clinically meaningful blood concentrations when delivered in two different physical configurations, with a median Tmax of 13.5 minutes and 22.5 minutes, respectively. Part 1 also demonstrated arithmetic mean maximum concentrations (Cmax) of 771 pg/mL and 580 pg/mL for the two configurations, or geometric mean Cmax values of 258pg/mL and 268pg/mL for the two configurations, respectively. These geometric mean Cmax and median Tmax values are consistent with those previously reported for approved injectable epinephrine devices such as EpiPen®.
As shown below, the healthy volunteers were also exposed to a 0.5mg intramuscular injection (IM) of epinephrine, allowing for a comparison with the pharmacokinetics, safety, and tolerability of the higher end of the approved dosage range of epinephrine, consistent with guidance received from the U.S. Food and Drug Administration (FDA) in a written response to the company’s pre-Investigational New Drug (pre-IND) submission.
The findings show that these two configurations of the selected AQST-109 formulation can deliver clinically meaningful blood concentrations of epinephrine sooner than that observed with the higher dose of epinephrine intramuscular injection, and in line with existing epinephrine autoinjectors, and without concerns for safety or tolerability.
“We are very pleased with the results from the first part of this study. We again showed pharmacokinetic results for AQST-109 that demonstrate delivery of epinephrine with the speed and absorption necessary for a rescue product,” said Keith Kendall, Chief Executive Officer of Aquestive. “With so many patients, for a variety of reasons, not having their rescue medication where they need it, when they need it, we believe AQST-109 can significantly improve how patients and caregivers manage anaphylaxis and look forward to our continued development in the next part of the EPIPHAST study.”
“Anaphylaxis is a disease state that has faced a lack of innovation for decades. Epinephrine has been in use for more than a century, yet epinephrine auto-injectors are often underutilized due to various factors, including needle phobia, delayed administration, and failure to carry,” said John Oppenheimer, M.D., FAAAAI, Clinical Professor of Medicine at UMDNJ Rutgers, Pulmonary and Allergy Associates NJ. “AQST-109 shows promise as a novel alternative for the treatment of anaphylaxis, given the small size, portability, and needleless delivery of the product. I look forward to further evaluation of this investigational medicine.”
Aquestive has commenced Part 2 of the EPIPHAST study and expects to report topline results in the first half of 2022. Part 2 is a randomized, crossover design comparing AQST-109 12mg to epinephrine IM 0.3mg.
About Anaphylaxis
Anaphylaxis is a potentially life-threatening systemic allergic reaction, with an estimated incidence of 50 to 112 episodes per 100,000 people per year. The frequency of hospital admissions for anaphylaxis has increased 500-700% in the last 10-15 years. The most common causes of reactions that can include anaphylaxis are medications, foods (such as peanuts), and venom from insect stings. Epinephrine injection is the current standard of treatment intended to reverse the potentially severe manifestation of anaphylaxis, which may include red rash, throat swelling, respiratory problems, gastrointestinal distress, and loss of consciousness.About AQST-109
AQST-109 is a polymer matrix-based epinephrine prodrug administered as a sublingual film that is applied under the tongue for the rapid delivery of epinephrine. The product is similar in size to a postage stamp, weighs less than an ounce, and begins to dissolve on contact. No water or swallowing is required for administration. The packaging for AQST-109 is thinner and smaller than an average credit card, can be carried in a pocket, and is designed to withstand weather excursions such as exposure to rain and/or sunlight.About Aquestive Therapeutics
Aquestive Therapeutics is a pharmaceutical company advancing medicines to solve patients’ problems with current standards of care and provide transformative products to improve their lives. The Company has four approved and licensed products, and commercialized one internally-developed proprietary product to date, Sympazan® (clobazam) oral film. The Company also has a commercial proprietary product pipeline focused on the treatment of diseases of the central nervous system, or CNS, and other unmet needs, and is developing orally administered complex molecules to provide alternatives to invasively administered standard of care therapies. The Company also collaborates with other pharmaceutical companies to bring new molecules to market using proprietary, best-in-class technologies, like PharmFilm®, and has proven capabilities for drug development and commercialization.Forward-Looking Statement
Certain statements in this press release include “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “believe,” “anticipate,” “plan,” “expect,” “estimate,” “intend,” “may,” “will,” or the negative of those terms, and similar expressions, are intended to identify forward-looking statements. These forward-looking statements include, but are not limited to, statements regarding the advancement and related timing of AQST-109 through the regulatory and development pipeline and clinical and business strategies, market opportunities, and other statements that are not historical facts. These forward-looking statements are subject to the uncertain impact of the COVID-19 global pandemic on our business including with respect to our clinical trials including site initiation, patient enrollment and timing and adequacy of clinical trials; on regulatory submissions and regulatory reviews and approvals of our product candidates; pharmaceutical ingredient and other raw materials supply chain, manufacture, and distribution; sale of and demand for our products; our liquidity and availability of capital resources; customer demand for our products and services; customers’ ability to pay for goods and services; and ongoing availability of an appropriate labor force and skilled professionals. Given these uncertainties, the Company is unable to provide assurance that operations can be maintained as planned prior to the COVID-19 pandemic.These forward-looking statements are based on our current expectations and beliefs and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Such risks and uncertainties include, but are not limited to, risks associated with the Company’s development work, including any delays or changes to the timing, cost and success of our product development activities and clinical trials for AQST-109 and our other product candidates; risk of delays in FDA approval of AQST-109, Libervant™ (diazepam) Buccal Film and our other drug candidates or failure to receive FDA approval; ability to address the concerns identified in the FDA’s Complete Response Letter dated September 25, 2020 regarding the New Drug Application for Libervant; risk of our ability to demonstrate to the FDA “clinical superiority” within the meaning of the FDA regulations of Libervant relative to FDA-approved diazepam rectal gel and nasal spray products including by establishing a major contribution to patient care within the meaning of FDA regulations relative to the approved products as well as risks related to other potential pathways or positions which are or may in the future be advanced to the FDA to overcome the seven year orphan drug exclusivity granted by the FDA for the approved nasal spray product of a competitor in the U.S. and there can be no assurance that we will be successful; risk that a competitor obtains FDA orphan drug exclusivity for a product with the same active moiety as any of our other drug products for which we are seeking FDA approval and that such earlier approved competitor orphan drug blocks such other product candidates in the U.S. for seven years for the same indication; risk in obtaining market access for other reasons; risk inherent in commercializing a new product (including technology risks, financial risks, market risks and implementation risks and regulatory limitations); risk of development of our sales and marketing capabilities; risk of sufficient capital and cash resources, including access to available debt and equity financing and revenues from operations, to satisfy all of our short-term and longer term liquidity and cash requirements and other cash needs, at the times and in the amounts needed; risks related to the outsourcing of certain marketing and other operational and staff functions to third parties; risk of the rate and degree of market acceptance of our product and product candidates; the success of any competing products, including generics; risk of the size and growth of our product markets; risks of compliance with all FDA and other governmental and customer requirements for our manufacturing facilities; risks associated with intellectual property rights and infringement claims relating to the Company’s products; risk of unexpected patent developments; the impact of existing and future legislation and regulatory provisions on product exclusivity; legislation or regulatory actions affecting pharmaceutical product pricing, reimbursement or access; claims and risks that may arise regarding the safety or efficacy of the Company's products and product candidates; risk of loss of significant customers; risks related to legal proceedings and associated costs, including patent infringement, investigative and antitrust litigation matters; changes in government laws and regulations; risk of product recalls and withdrawals; uncertainties related to general economic, political, business, industry, regulatory and market conditions and other unusual items; and other uncertainties affecting the Company described in the “Risk Factors” section and in other sections included in our Annual Report on Form 10-K, in our Quarterly Reports on Form 10-Q, and in our Current Reports on Form 8-K filed with the Securities and Exchange Commission. Given those uncertainties, you should not place undue reliance on these forward-looking statements, which speak only as of the date made. All subsequent forward-looking statements attributable to us or any person acting on our behalf are expressly qualified in their entirety by this cautionary statement. The Company assumes no obligation to update forward-looking statements or outlook or guidance after the date of this press release whether as a result of new information, future events or otherwise, except as may be required by applicable law.
PharmFilm®, Sympazan® and the Aquestive logo are registered trademarks of Aquestive Therapeutics, Inc. All other registered trademarks referenced herein are the property of their respective owners.
Investor Inquiries
ICR Westwicke
Stephanie Carrington
Stephanie.carrington@westwicke.com
646-277-1282A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f6674799-ad9d-4eec-8253-29861728ed69